
20대/여자
일주일 전 왼쪽 옆구리 통증, 식은땀, 오한
**병원 ER에서 abdomen enhanced CT 촬영함
'신우신염까지는 아니고 요로감염인 것 같다'는 말 듣고
ciprofloxacin 500 mg bid X3일 처방 받음.
이후 진통소염제를 복용함에도 밤마다 오한이 있어 내원하였습니다.
항생제는 3일 복용하고 중단한 상태
다시 의료기관 방문하지 않음.
Lt. Knocking Td는 저명하지 않음.
Q.
1. 신우신염은 아닌데 열이 났었다면 ureteritis 정도였을까?
2. 왼쪽 옆구리 통증이 있었으므로 신우신염이었지 않았을까?
3. 병소는 다른 곳이고 우연히 검사결과가 요로감염 소견이지 않았을까?
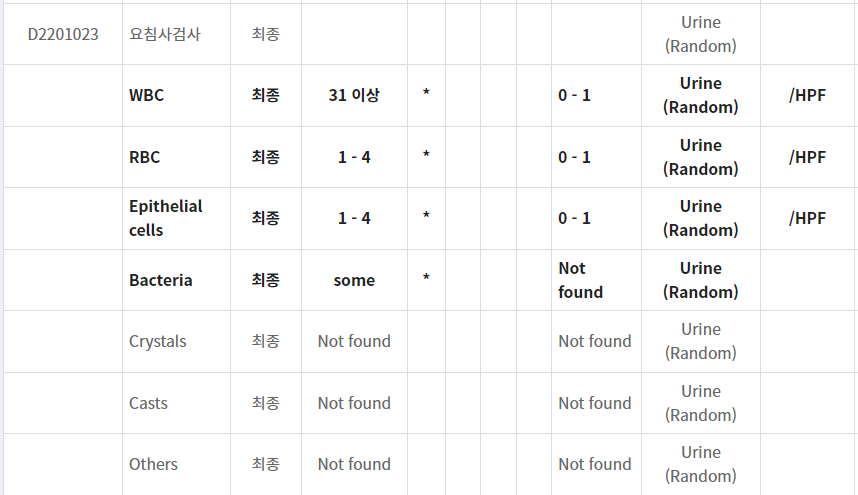
plan) blood test and urine test
Rx) amoxicillin 375 mg tid + doxycycline 100 mg bid
acetaminophen 650 mg, 2T tid
DDx
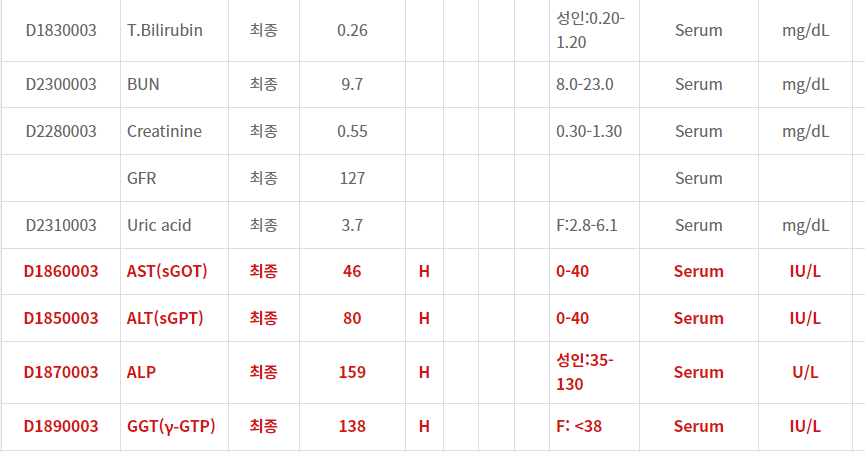
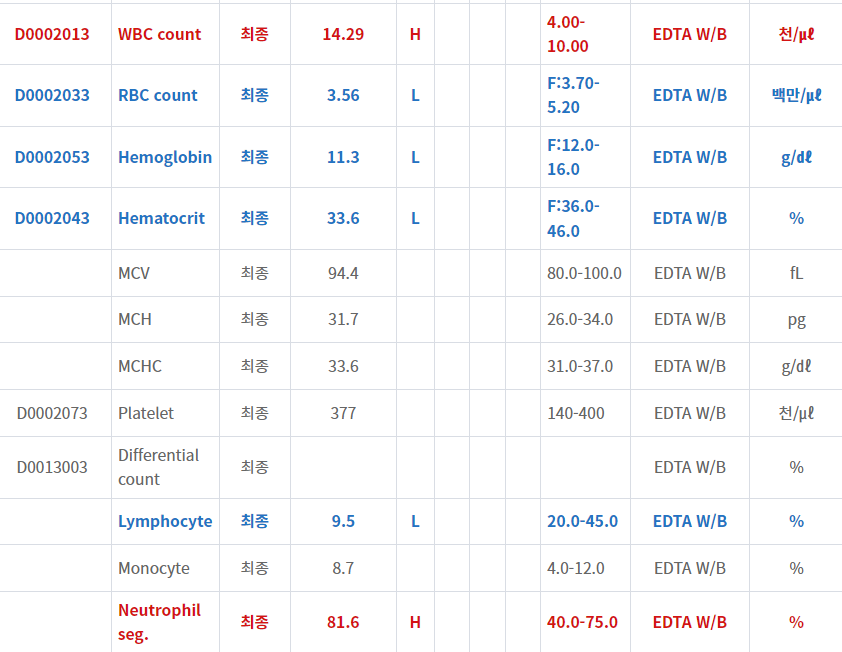
- Pyelonephritis? Liver enzyme은 secondary change?
- Liver abscess? 요로감염은 incidental finding?
Q.
신우신염은 아닌데 열이 났었다면 ureteritis 정도였을까?
왼쪽 옆구리 통증이 있었으므로 신우신염이었지 않았을까?
병소는 다른 곳이고 우연히 요로감염 소견이 있지 않았을까?
- CT를 최근에 찍었지만 **병원이 멀리 있어서 CT 가져 오기도 쉽지 않고
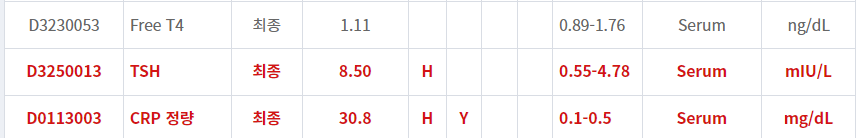
- 현재 일주일 넘게 해열제 복용에도 불구하고 오한이 지속되며 (WBC, CRP 증가가 많이 되어 있고)
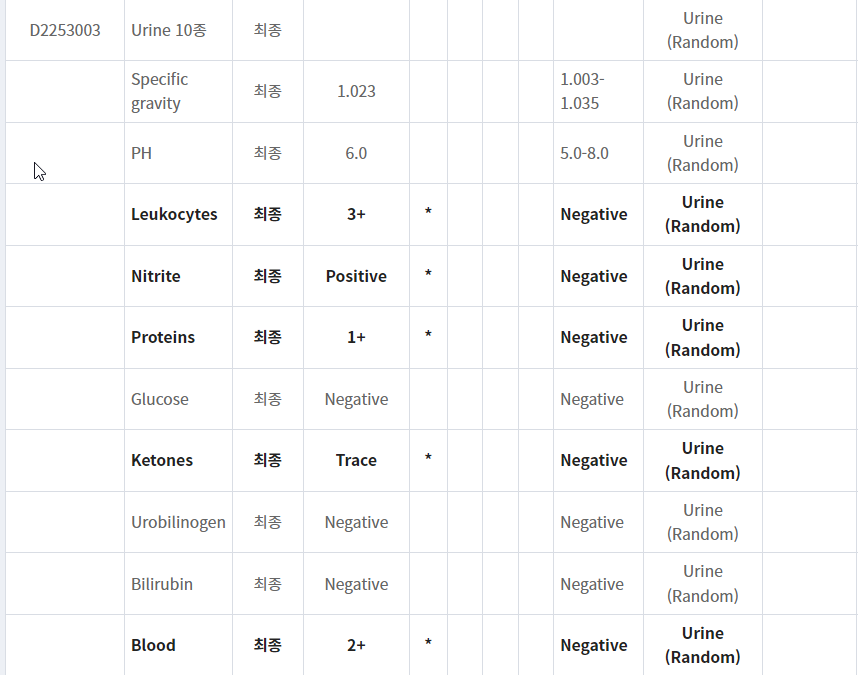
- 일주일 전 CT에서 신우신염까지는 아니었다고 하였음에도 pyuria, bacteriuria 소견 보이고
- liver abscess 등 다른 발열 원인 감별도 필요하므로 복부 CT 다시 찍기로 함.
Plan) abdomen CT, enhanced
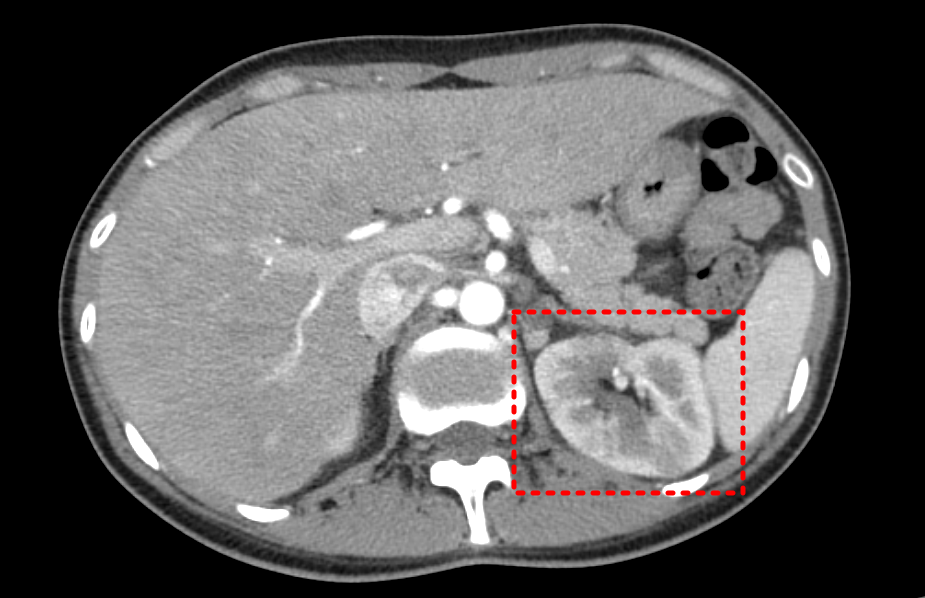
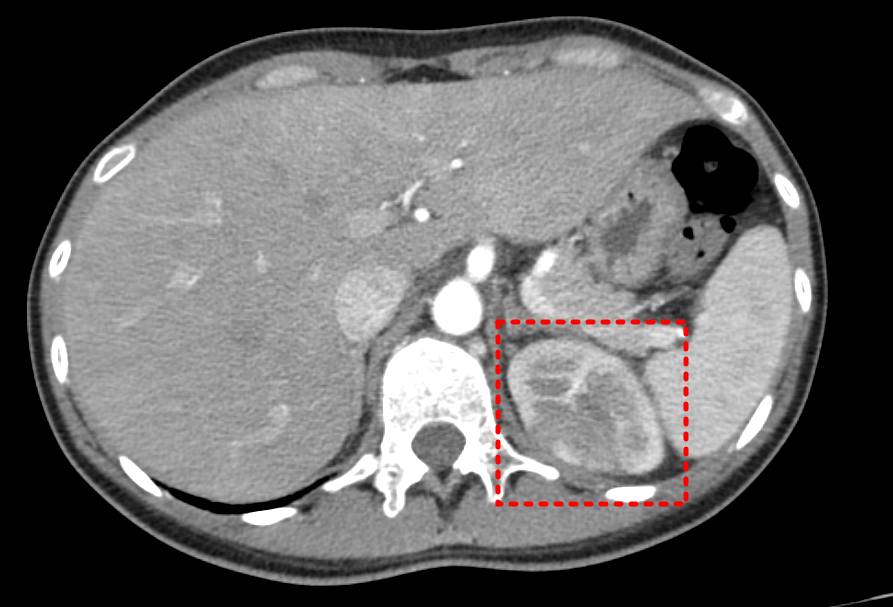
Dx ) Acute pyelonephritis, Lt
Plan) Doxycycline stop
Amoxicillin/Clavulanate 유지 (ciprofloxacin 3일 사용 후 중단된 상태이고 기왕 amoxicillin/clavulanate 2일 복용 중이면서 증상 호전이 있으므로)
Empiric antimicrobial agent selection for acute complicated urinary tract infection
|
Patient population
|
Risk for MDR?*
|
Empiric regimens
|
Comments
|
|
Hospitalized with:
|
N/A
|
|
|
|
Other hospitalized patients
|
No
|
|
|
|
Yes
|
|
|
|
|
Outpatients
|
No, and no concerns with fluoroquinolones (eg, at low risk for adverse effects)
|
|
|
|
No, but with concerns with fluoroquinolones (eg, at risk for adverse effects)
|
|
|
|
|
Yes
|
|
|
These antibiotic regimens represent our approach to empiric treatment for acute complicated UTI. Once culture and susceptibility testing results are available, the regimen should be tailored to those results. If feasible, an antibiotic with a narrow spectrum of activity should be chosen to complete the antibiotic course.
MDR: multidrug resistance; IV: intravenous; VRE: vancomycin-resistant Enterococcus; MRSA: methicillin-resistant Staphylococcus aureus; IM: intramuscular; TMP-SMX: trimethoprim-sulfamethoxazole; UTI: urinary tract infection.
* Risk factors for MDR gram-negative UTIs include any one of the following in the prior three months:
- An MDR, gram-negative urinary isolate, including a fluoroquinolone-resistant Pseudomonas urinary isolate
- Inpatient stay at a health care facility (eg, hospital, nursing home, long-term acute care facility)
- Use of a fluoroquinolone, TMP-SMX, or broad-spectrum beta-lactam (eg, third- or later-generation cephalosporin)
- Travel to parts of the world with high rates of MDR organisms
Ref. UpToDate 2023.01.05
'감염내과 > 요로감염' 카테고리의 다른 글
| 급성 신우신염 치료, MDR 감염 위험이 낮은 경우 (0) | 2023.02.17 |
|---|---|
| Methenamine Hippurate는 여성에서 재발성 요로감염을 예방한다. (0) | 2023.01.24 |
| ESBL 양성 대장균, 급성 방광염, 경구 항생제 선택 (0) | 2022.04.18 |
| 임신부 무증상 세균뇨, 추적검사와 치료는 어떻게 하나요? (0) | 2021.05.29 |
| 임신부 무증상 세균뇨, 왜 치료해야 하나요? 그리고 항생제 (0) | 2021.05.29 |



