
'저는 삭센다 맞으면 설사를 아주 심하게 해요'
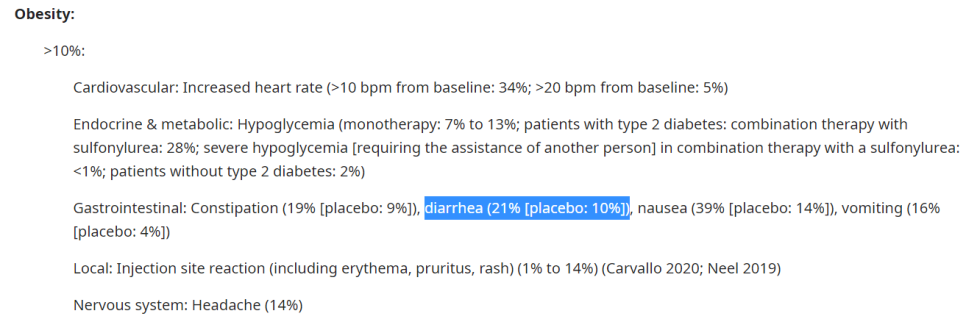
GLP-1 receptor agonists의 이상 반응은 주로 위장관, 특히 오심, 구토, 설사이며 시험마다 다르지만 10-50 % 정도에서 있습니다.
|
Gastrointestinal In a meta-analysis of 17 randomized trials comparing subcutaneously administered GLP-1 receptor agonists (exenatide, liraglutide, albiglutide, taspoglutide, lixisenatide) with placebo or an active comparator (insulin glargine, dipeptidyl peptidase 4 [DPP-4] inhibitor, thiazolidinedione, sulfonylurea) in patients with type 2 diabetes and suboptimal control on one or two oral agents, patients randomly assigned to treatment with GLP-1 receptor agonists compared with placebo or active comparator experienced more nausea (8 to 40 percent more), diarrhea (3 to 118 percent more), and weight loss (-1.3 to -5.1 kg). Subcutaneous and oral semaglutide are also associated with gastrointestinal side effects. In one trial, nausea, vomiting, and diarrhea occurred in 15, 9, and 12.3 percent, respectively, of patients taking semaglutide (14 mg orally daily) compared with 6.9, 4.1, and 7.9 percent, respectively, of patients taking sitagliptin (100 mg daily). Nausea may wane with duration of therapy and can be reduced with dose titration. Nausea is the most frequent adverse event with exenatide once weekly, but it has been reported less frequently with once-weekly than with twice-daily administration (26 versus 50 percent) and also less frequently than with liraglutide (9 versus 21 percent). |
REF. UpToDate 2020.11.03
'내분비내과 > 비만' 카테고리의 다른 글
| 비만치료제 삭센다의 흔한 이상 반응 (0) | 2021.06.10 |
|---|---|
| [비만치료제] 콘트라브 (부프로피온-날트렉손), Contrave (bupropion-naltrexone) (0) | 2021.01.29 |
| [비만치료제] GLP-1 수용체 효능제, 삭센다 (0) | 2021.01.29 |
| [비만치료제] 오르리스타트, Orlistat (0) | 2021.01.29 |
| 비만에서 수술 적응증, Bariatric operations for management of obesity (0) | 2018.01.15 |


