Nonmetastatic, locally advanced, unresectable & borderline resectable, exocrine pancreatic cancer 1
국소적으로 진행되어 수술을 바로 시행하기 어려운 췌장암에서 수술 전 항암제를 투약하는데
Neoadjuvant chenotherapy
그 이유는 다음과 같습니다.
① 수술의 주된 목표인 margin-negative resection 비율을 높이기 위해
② Micrometastatic disease 치료를 위해
③ 수술이 생존 이득을 제공하지 않을 것 같은 환자 선택하기 개선하기 위해 (수술 전 전이가 빠르게 진행하는 환자)
지금 절제하기는 어렵지만 항암치료를 하여 크기를 줄여서 수술을 용이하게 하고, 영상검사에서 보이지 않은 전이 병변을 제거하기 위함입니다. 아래의 접근 방법은 the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology (ESMO)와 일치합니다.
초기 치료 선택에 영향을 줄 수 있는 germline molecular alterations 또는 somatic molecular alterations에 대한 지식 이외에도 중요한 2가지 고려사항이 있습니다.
1. 조직 진단이 치료 시작 이전에 있어야 합니다. 이것은 조직 진단이 필요하지 않을 수도 있는 수술 우선 치료 전략과는 다릅니다. 수술이 우선 고려되는 경우에는 수술을 하면 조직 검사 결과를 알 수 있지만 수술 전 항암치료 (neoadjuvant CTx)를 하는 경우에는 조직 진단이 먼저 있어야 하고 transcutaneous 또는 EUS-FNA로 조직을 얻습니다.
2. 폐쇄성 황달로 내원한 환자의 경우에 neoadjuvant therapy의 전달을 위해 많게는 6개월까지 지속적인 biliary decompression을 필요로 합니다. 이것은 보통 biliary stent가 이용됩니다.
Treatment algorithm for nonmetastatic exocrine pancreatic cancer
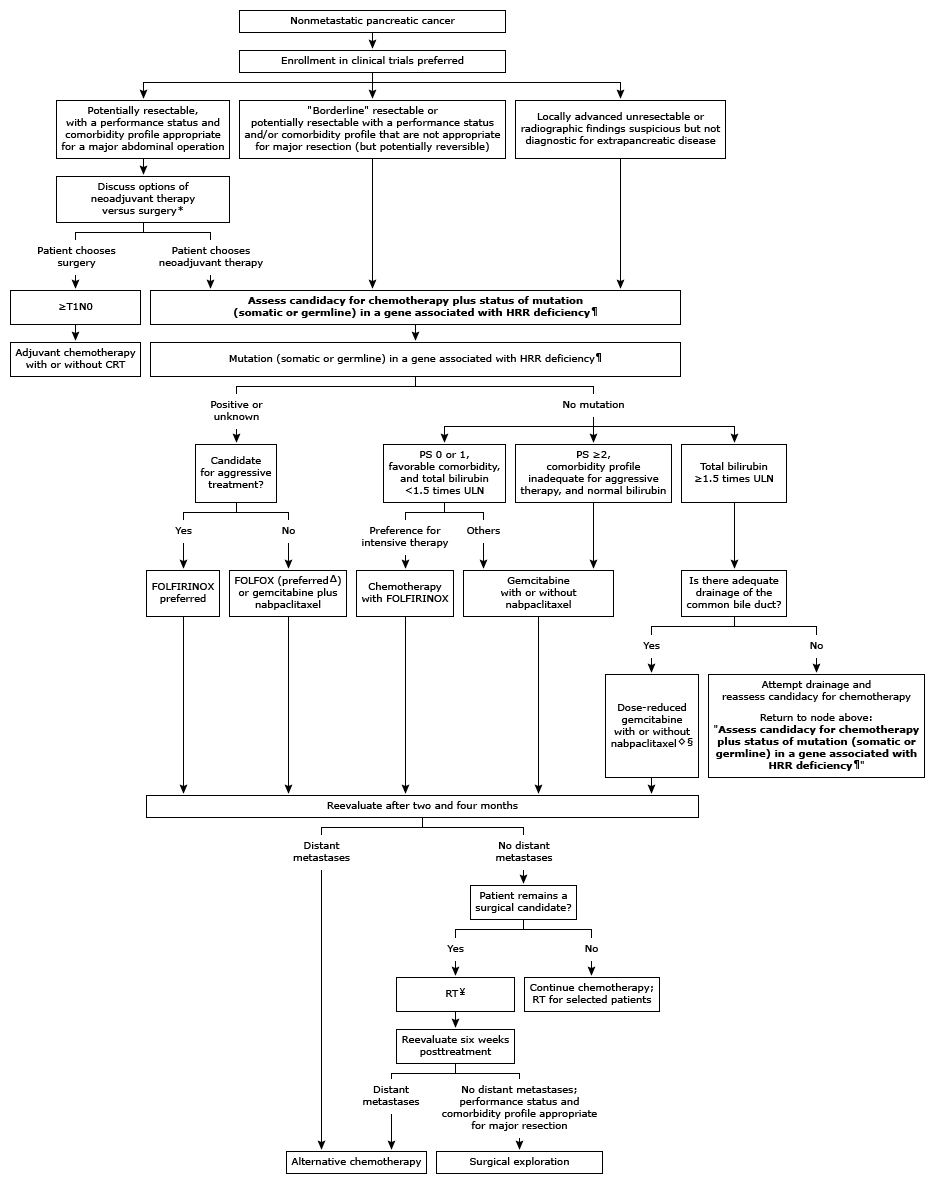
HRR: homologous recombination repair; CRT: chemoradiotherapy; PS: Eastern Cooperative Oncology Group performance status; ULN: upper limit of normal; FOLFIRINOX: oxaliplatin plus irinotecan with leucovorin and short-term infusional fluorouracil; FOLFOX: leucovorin plus short-term infusional fluorouracil and oxaliplatin; nabpaciltaxel: nanoparticle albumin-bound paclitaxel; RT: radiation therapy; BRCA: breast cancer susceptibility gene; PALB2: partner and localizer of BRCA2.
* Although the available supporting data for neoadjuvant therapy in patients with potentially resectable disease are limited, given the overall poor prognosis even after complete resection and adjuvant therapy, we consider neoadjuvant therapy to be a reasonable alternative to upfront surgery, as long as performance status and comorbidity are sufficient to tolerate treatment. However, for the rare patient with a <2 cm apparently node-negative tumor (as determined by pretreatment imaging), neoadjuvant therapy is probably not warranted.
¶ Genes associated with HRR deficiency include BRCA1/2, PALB2, ATM, BAP1, BARD1, BLM, BRIP1, CHEK2, FAM175A, FANCA, FANCC, NBN, RAD50, RAD51, RAD51C, and RTEL1.
Δ Irinotecan could be added to later cycles if mutations are discovered in BRCA or PALB2 and the patient has tolerated FOLFOX adequately.
◊ Many clinicians would not administer gemcitabine for a total bilirubin above 2.5 ng/mL.
§ If poor performance status is due to recent infection (eg, cholangitis) and the patient is recovering well after intervention, dose-adjusted combination chemotherapy is preferred over single-agent gemcitabine. If performance status is poor due to locally advanced disease causing disabling pain and/or gastric outlet obstruction, aggressive symptom management should be undertaken prior to initiating chemotherapy.
¥ At some institutions, patients with a very good response to neoadjuvant chemotherapy would be taken directly to surgical exploration. Fluorouracil-based CRT (or stereotactic body RT, refer to UpToDate text) should be considered if further tumor shrinkage might increase the chance of a microscopically complete (R0) resection. It is unknown whether RT contributes to the R0 resection rate after chemotherapy (especially for combination regimens such as FOLFIRINOX). Another alternative is two to three months of additional chemotherapy alone.
|
Nonmetastatic, locally advanced, unresectable & borderline resectable, exocrine pancreatic cancer 1. 이 경우 neoadjuvant Tx는 radiation therapy (RT) 또는 chemoradiotherapy (CRT)보다는 chemotherapy를 제안합니다. 2. 최적 regimen은 정해지지 않았지만 임상 시험에 등록하는 것이 선호됩니다. 3. Mutation이 알려진 환자 또는 mutation 상태를 알 수 없거나 미결인 환자에서, performance status와 comorbidity profile이 괜찮고 적극적인 치료를 원하는 경우 FOLFIRINOX 병합 항암 치료가 선호됩니다 대부분의 환자에서 더 낮은 irinotecan 용량을 사용하는 modified FOLFIRINOX regimen을 선호합니다. * 여기서 FOLFIRINOX가 의미하는 것은 다음과 같습니다. FOL – folinic acid (also called leucovorin, calcium folinate or FA) F – fluorouracil (also called 5FU) Irin – irinotecan Ox – oxaliplatin |
Modified FOLFIRINOX chemotherapy for pancreatic cancer[1,2]
|
Cycle length: 14 days. |
|||
|
Drug |
Dose and route |
Administration |
Given on days |
|
Oxaliplatin* |
85 mg/m2 IV |
Dilute in 500 mL D5W¶ and administer over two hours (prior to leucovorin). Shorter oxaliplatin administration schedules (eg, 1 mg/m2 per minute) appear to be safe.[3] |
Day 1 |
|
LeucovorinΔ◊ |
400 mg/m2 IV |
Dilute in 250 mL NS or D5W¶ and administer over two hours (after oxaliplatin). |
Day 1 |
|
Irinotecan§ |
150 mg/m2 IV |
Dilute in 500 mL NS or D5W¶ and administer over 90 minutes. Administer concurrent with the last 90 minutes of leucovorin infusion, in separate bags, using a Y-line connection. |
Day 1 |
|
Fluorouracil (FU) |
2400 mg/m2 IV |
Dilute in 500 to 1000 mL 0.9% NS or D5W¶ and administer as a continuous IV infusion over 46 hours. To accommodate an ambulatory pump for outpatient treatment, can be administered undiluted (50 mg/mL) or the total dose diluted in 100 to 150 mL NS.¶ |
Day 1 |
|
Pretreatment considerations: |
|||
|
Emesis risk |
HIGH (greater than 90% frequency of emesis).¥ Refer to UpToDate topic on "Prevention and treatment of chemotherapy-induced nausea and vomiting in adults". |
||
|
Prophylaxis for infusion reactions |
Although infusion reactions have been reported with oxaliplatin, there is no recommended standard premedication for this regimen. Refer to UpToDate topic on "Infusion reactions to systemic chemotherapy". |
||
|
Vesicant/irritant properties |
Oxaliplatin and FU are irritants, but oxaliplatin can cause significant tissue damage; avoid extravasation. Refer to UpToDate topic on "Extravasation injury from chemotherapy and other non-antineoplastic vesicants". |
||
|
Infection prophylaxis |
Primary prophylaxis with G-CSF is not warranted. However, given the risk of grade 3 or 4 neutropenia (46%[1]), primary prophylaxis with G-CSF is used at many institutions, especially when this regimen is used in the adjuvant setting.[2] Refer to UpToDate topic on "Use of granulocyte colony stimulating factors in adult patients with chemotherapy-induced neutropenia and conditions other than acute leukemia, myelodysplastic syndrome, and hematopoietic cell transplantation". |
||
|
Dose adjustment for baseline liver or renal dysfunction |
A lower starting dose of oxaliplatin and irinotecan may be needed for severe renal insufficiency.[4,5] A lower starting dose of irinotecan and FU may be needed for patients with hepatic impairment.[5,6] NOTE: We do not recommend administration of FOLFIRINOX unless serum bilirubin is normal. Refer to UpToDate topics on "Chemotherapy hepatotoxicity and dose modification in patients with liver disease" and "Chemotherapy nephrotoxicity and dose modification in patients with renal insufficiency: Conventional cytotoxic agents". |
||
|
Maneuvers to prevent neurotoxicity |
Pharmacologic methods to prevent/delay the onset of oxaliplatin-related neuropathy are controversial due to the absence of large clinical trials proving benefit. Counsel patients to avoid exposure to cold during and for approximately 48 hours after each infusion.[4] Prolongation of the oxaliplatin infusion time from two to six hours may mitigate acute neurotoxicity. Refer to UpToDate topic on "Overview of neurologic complications of platinum-based chemotherapy". |
||
|
Cardiac issues |
QT prolongation and ventricular arrhythmias have been reported after oxaliplatin. ECG monitoring is recommended if therapy is initiated in patients with heart failure, bradyarrhythmias, coadministration of drugs known to prolong the QT interval, and electrolyte abnormalities. Avoid oxaliplatin in patients with congenital long QT syndrome. Correct hypokalemia and hypomagnesemia prior to initiating oxaliplatin. Cardiotoxicity observed with FU includes myocardial infarction/ischemia, angina, dysrhythmias, cardiac arrest, cardiac failure, sudden death, electrocardiographic changes, and cardiomyopathy. |
||
|
Monitoring parameters: |
|||
|
CBC with differential and platelet count prior to each treatment. |
|||
|
Electrolytes (especially potassium and magnesium) and liver and renal function prior to each treatment. |
|||
|
Irinotecan is associated with early and late diarrhea, both of which may be severe.[5] For patients who develop abdominal cramping and/or diarrhea within 24 hours of receiving irinotecan, administer atropine (0.3 to 0.6 mg IV) and premedicate with atropine during later cycles. Patients must be instructed in the early use of loperamide for late diarrhea. Patients who develop diarrhea should be closely monitored and supportive care measures (eg, fluid and electrolyte replacement, loperamide, antibiotics, etc) should be provided as needed. Refer to UpToDate topic on "Enterotoxicity of chemotherapeutic agents". |
|||
|
Assess changes in neurologic function prior to each treatment. |
|||
|
Suggested dose modifications for toxicity: |
|||
|
Myelotoxicity |
Do not retreat unless neutrophil count is ≥1500/microL and platelets are ≥75,000/microL. The following dose reduction guidelines for hematologic toxicity are recommended; several of these are based upon recommendations in the original FOLFIRINOX protocol.[7] Neutropenia If day 1 treatment delayed for granulocytes is <1500/microL or febrile neutropenia or grade 4 neutropenia >7 days: Reduce irinotecan dose to 120 mg/m2. For second occurrence: Reduce oxaliplatin dose to 60 mg/m2. If nonrecovery after a two-week delay, or if there is a third occurrence of granulocytes <1500/microL on day 1, discontinue treatment. For grade 4 neutropenia >7 days during treatment or febrile neutropenia, reduce oxaliplatin dose to 60 mg/m2 and the infusional FU dose to 75% of the original dose. For the second occurrence, reduce dose of irinotecan to 120 mg/m2 and the dose of infusional FU an additional 25%. Discontinue treatment for third occurrence. Thrombocytopenia If day 1 treatment delayed for platelet count <75,000/microL, reduce oxaliplatin dose to 60 mg/m2 and reduce the continuous infusion FU to 75% of original doses. For second occurrence, reduce irinotecan dose to 120 mg/m2. If nonrecovery after a two-week delay, or if there is a third occurrence of platelets <75,000/microL, discontinue treatment. For grade 3 or 4 thrombocytopenia during treatment, reduce oxaliplatin dose to 60 mg/m2 and the infusional FU dose to 75% of the original dose. For the second occurrence, reduce dose of irinotecan to 120 mg/m2 and the dose of infusional FU an additional 25%. Discontinue treatment for third occurrence. |
||
|
Diarrhea |
Do not retreat with FOLFIRINOX until resolution of diarrhea for at least 24 hours without antidiarrheal medication. For diarrhea grade 3 or 4, or diarrhea with fever and/or grade 3 or 4 neutropenia, reduce irinotecan dose to 120 mg/m2. For second occurrence, reduce the oxaliplatin dose to 60 mg/m2 and the continuous FU dose to 75% of original dose. Discontinue treatment for third occurrence. NOTE: Severe diarrhea, mucositis, and myelosuppression after FU should prompt evaluation for DPD deficiency. Refer to UpToDate topic on "Enterotoxicity of chemotherapeutic agents." |
||
|
Mucositis or hand-foot syndrome |
For grade 3 to 4 toxicity, reduce dose of infusional FU by 25%. |
||
|
Pulmonary toxicity |
Oxaliplatin has rarely been associated with pulmonary toxicity. Withhold oxaliplatin for unexplained pulmonary symptoms until interstitial lung disease or pulmonary fibrosis is excluded. Refer to UpToDate topic on "Pulmonary toxicity associated with antineoplastic therapy: Cytotoxic agents". |
||
|
Neurologic toxicity |
For persistent grade 3 paresthesias/dysesthesias or transient grade 2 symptoms lasting >7 days, decrease oxaliplatin dose by 25%.[4] Discontinue oxaliplatin for grade 4 or persistent grade 3 paresthesia/dysesthesia. There is no recommended dose for resumption of FU administration following development of hyperammonemic encephalopathy, acute cerebellar syndrome, confusion, disorientation, ataxia, or visual disturbances; the drug should be permanently discontinued.[6] |
||
|
Cardiotoxicity |
Cardiotoxicity observed with FU includes myocardial infarction/ischemia, angina, dysrhythmias, cardiac arrest, cardiac failure, sudden death, ECG changes, and cardiomyopathy. There is no recommended dose for resumption of FU administration following development of cardiac toxicity, and the drug should be discontinued.[6] |
||
|
Other toxicity |
Any other toxicity ≥grade 2, except anemia and alopecia, can justify dose reduction if medically indicated. For other nonhematologic toxicities, if grade 2, hold treatment until ≤grade 1; if grade 3 or 4, hold treatment until ≤grade 2.[5] |
||
|
If there is a change in body weight of at least 10%, doses should be recalculated. |
|||
This table is provided as an example of how to administer this regimen; there may be other acceptable methods. This regimen must be administered by a clinician trained in the use of chemotherapy, who should use independent medical judgment in the context of individual circumstances to make adjustments, as necessary.
IV: intravenous; D5W: 5% dextrose in water; NS: normal saline; G-CSF: granulocyte colony-stimulating factors; QT: time between the start of the Q wave and the end of the T wave (heart electrical cycle); ECG: electrocardiogram; CBC: complete blood count; DPD: dihydropyrimidine dehydrogenase.
* Many centers routinely infuse oxaliplatin via central venous line because of local pain with infusion into a peripheral vein.
¶ Diluent solutions should not be modified without consulting a detailed reference due to potential incompatibility(ies).
Δ Leucovorin dose is given for d,l-racemic mixture.[8] Use half the dose for LEVOleucovorin (l-leucovorin).
◊ In the setting of advanced (metastatic) disease, day 1 leucovorin, which was not administered in one of the supporting studies,[1] is optional.[5] Whether a reduced starting dose is needed in patients who are homozygous for the UGT 1A1*28 allele (Gilbert syndrome) and whether testing for this allele should be carried out prior to starting irinotecan is controversial. Refer to UpToDate topic on "Enterotoxicity of chemotherapeutic agents".
§ A lower initial dose of irinotecan may be considered for patients with poor performance status, prior pelvic or abdominal radiotherapy, or increased bilirubin levels.
¥ At many institutions, regimens that combine oxaliplatin with irinotecan on day 1 are considered highly emetogenic, warranting the use of a neurokinin-1 receptor antagonist on day 1. The National Comprehensive Cancer Network considers this and similar regimens as moderately emetogenic.
|
4. performance status와 comorbidity profile이 borderline인 경우에는 이리노테칸을 제외한 FOLFOX로 시작하고, mutation이 HRR deficiency와 관련된 유전자에서 발견되고 환자고 FOLFOX에 잘 견딘다면 이리노테칸은 이후 cycle에서 추가될 수 있습니다. 5. Germline 또는 tumor에서 HRR pathway alterations이 없는 환자의 경우 gemcitabine plus nanoparticle albumin-bound paclitaxel (nabpaclitaxel) 또는 FOLFIRINOX입니다. 6. Gemcitabine 단일 약제도 사용될 수 있지만 locally advanced setting에서 절제로의 전환이 5 % 미만밖에 되지 않으므로 이 치료는 잠재적으로 curative하다고 간주되지 않습니다. |
|
7. 절제 가능성에 대한 평가는 neoadjuvant treatment 4-6개월 이후에 이루어져야 합니다. FOLFIRINOX와 gemcitabine/nabpaclitaxel 이전 시대에는 gemcitabine 또는 chemoradiation 이후 수술적 절제를 하는 것이 매우 드물었습니다. 좀 더 최근에는 FOLFIRINOX 또는 gemcitabine/nabpaclitaxel regimens 이후에 수술적을 불가능하였던 암을 절제 가능한 상태로 전환시키는 것이 일반적이게 되었습니다 (정확한 전환 비율은 큰 다기관 ㅇ녀구에서 정의될 필요성은 있습니다). |
제가 전공의 때는 췌장암 항암치료제로 gemcitabine, gemcitabine-cisplatin 항암제를 사용하던 시대였고 결과는 좋지 않았습니다. FOLFOX가 아닌 FOLFIRINOX와 gemcitabine/nabpaclitaxel regimens은 분명히 없었던 것으로 기억합니다. 2014년 NEJM에 실린 전이성 췌장 종양에서 gemcitabine과 비교한 FOLFIRINOX 시험 결과입니다.
342명의 전이성 췌장암 환자들을 무작위 할당하려 FOLFIRINOX와 gemcitabine을 투약하였으며, 전체 생존기간 중간값은 젬시타빈 그룹은 6.8 개월, 폴피리녹스 그룹은 11.1 개월, Progression-free survival 중간값은 젬시타빈은 3.3개월, 폴피리녹스는 6.4 개월이었습니다.



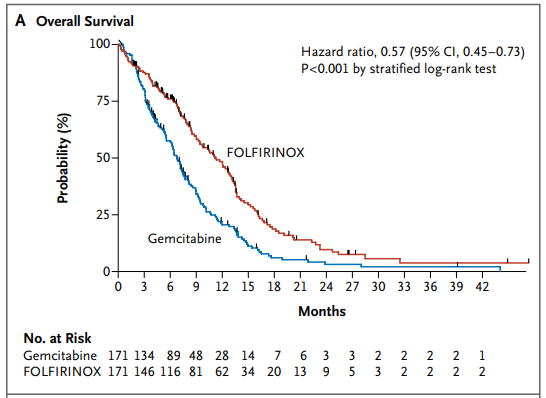
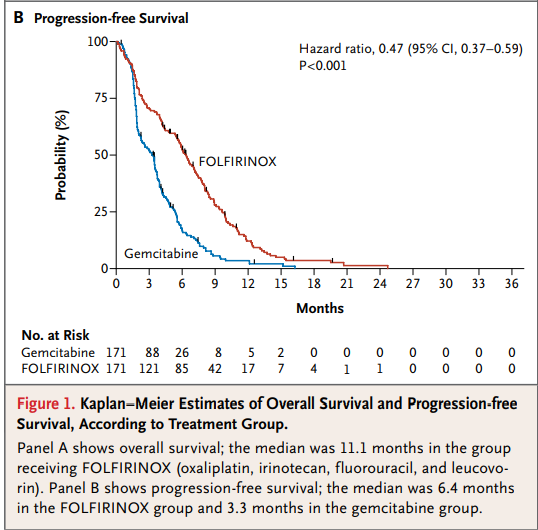



REF. UpToDate 2020.09.13
'소화기(췌장) > 췌장암' 카테고리의 다른 글
| 췌장암 임상발현에서 CA 19-9의 역할 ① (0) | 2021.04.25 |
|---|---|
| 새롭게 진단된 당뇨병 환자에서 췌장암 감시 검사 (0) | 2021.02.20 |
| 췌장암의 증상과 징후, Signs and symptoms associated with pancreatic cancer (0) | 2020.09.13 |
| [Pancreas] 췌장암, TNM staging AJCC UICC 8th edition (0) | 2020.09.01 |
| 췌장암 통증 [식후에 악화, 똑바로 누우면 악화, 밤에 악화 ↔ 구부려서 누우면 호전] (0) | 2020.08.30 |



