소라페닙 단독치료는 unresectable hepatocellular cancer의 표준 1차 치료제입니다. Child-Pugh A cirrhosis보다 나쁘지 않은 이전에 치료받지 않은 500명 이상의 환자를 대상으로 atezolizumab plus bevacizumab을 sorafenib과 비교한 시험에서 atezolizumab plus bevacizatezolimubaumab 그룹이 객관적 반응률이 2배였고 생존률과 PFS 모두 개선시켰습니다. 이 데이터에 근거하여 atezolizumab plus bevacizumab은 2020년 5월 29일 US FDA로부터 이전 전신 치료를 받지 않은 unresectable or metastatic HCC 환자를 위해 승인되었습니다.
Unresectable or metastatic HCC 환자에서 FDA에 승인된 1차 약제는 sorafenib, lenvatinib이었으나 atezolizumab plus bevacizatezolimubaumab이 추가되었습니다.
|
What's New First-line atezolizumab plus bevacizumab for unresectable hepatocellular cancer (May 2020) Sorafenib monotherapy represents a standard first-line regimen for unresectable hepatocellular cancer (HCC). In a trial comparing sorafenib with the combination of the immune checkpoint inhibitor atezolizumab plus bevacizumab in over 500 previously untreated patients with no worse than Child-Pugh A cirrhosis, the immunotherapy combination doubled the objective response rate and improved both survival and progression-free survival. Grade 3 or 4 adverse events occurred in more than 50 percent of patients in each group, but hypertension, pyrexia, transaminitis, and proteinuria were more frequent with combined therapy. Largely based on this study, the US Food and Drug Administration (FDA) approved atezolizumab plus bevacizumab for treatment of patients with unresectable or metastatic HCC who have not received prior systemic therapy. We reserve this approach for healthy patients with unresectable HCC who have no worse than Child-Pugh A cirrhosis, and who have not recurred following a liver transplantation (이식 후 재발한 경우에는 권고하지 않음). |

N Engl J Med 2020;382:1894-905.

|
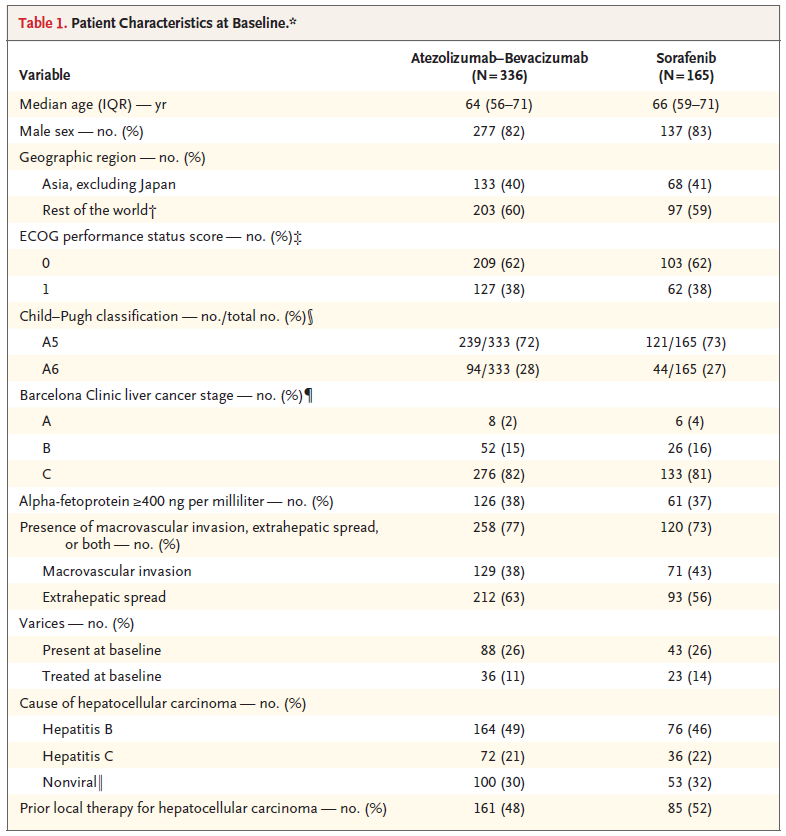
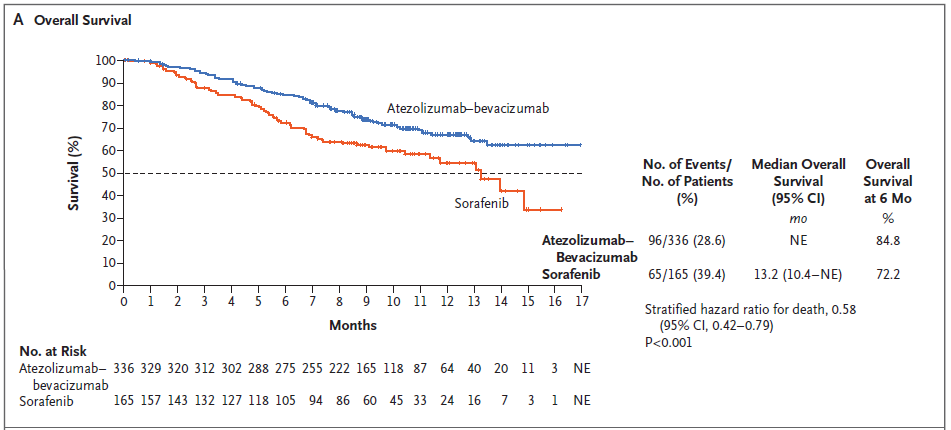
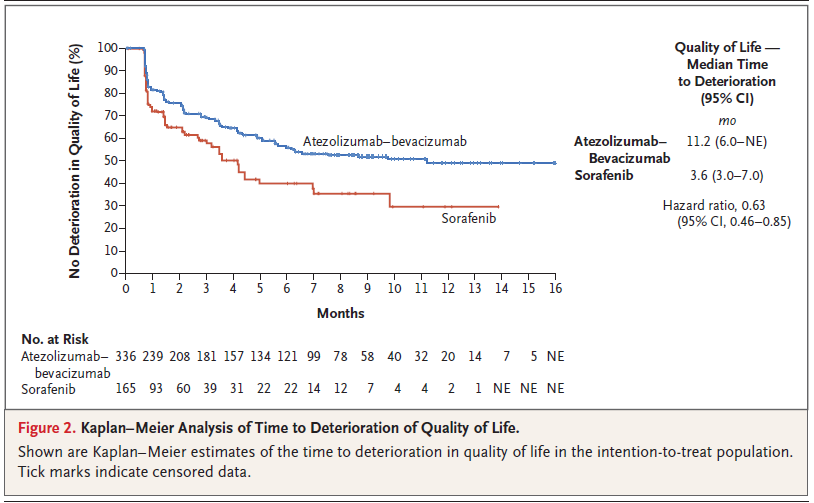
BACKGROUND The combination of atezolizumab and bevacizumab showed encouraging antitumor activity and safety in a phase 1b trial involving patients with unresectable hepatocellular carcinoma. METHODS In a global, open-label, phase 3 trial, patients with unresectable hepatocellular carcinoma who had not previously received systemic treatment were randomly assigned in a 2:1 ratio to receive either atezolizumab plus bevacizumab or sorafenib until unacceptable toxic effects occurred or there was a loss of clinical benefit. The coprimary end points were overall survival and progression-free survival in the intention-to-treat population, as assessed at an independent review facility according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). RESULTS The intention-to-treat population included 336 patients in the atezolizumab–bevacizumab group and 165 patients in the sorafenib group. At the time of the primary analysis (August 29, 2019), the hazard ratio for death with atezolizumab– bevacizumab as compared with sorafenib was 0.58 (95% confidence interval [CI], 0.42 to 0.79; P<0.001). Overall survival at 12 months was 67.2% (95% CI, 61.3 to 73.1) with atezolizumab–bevacizumab and 54.6% (95% CI, 45.2 to 64.0) with sorafenib. Median progression-free survival was 6.8 months (95% CI, 5.7 to 8.3) and 4.3 months (95% CI, 4.0 to 5.6) in the respective groups (hazard ratio for disease progression or death, 0.59; 95% CI, 0.47 to 0.76; P<0.001). Grade 3 or 4 adverse events occurred in 56.5% of 329 patients who received at least one dose of atezolizumab–bevacizumab and in 55.1% of 156 patients who received at least one dose of sorafenib. Grade 3 or 4 hypertension occurred in 15.2% of patients in the atezolizumab–bevacizumab group; however, other high-grade toxic effects were infrequent. CONCLUSIONS In patients with unresectable hepatocellular carcinoma, atezolizumab combined with bevacizumab resulted in better overall and progression-free survival outcomes than sorafenib. (Funded by F. Hoffmann–La Roche/Genentech; ClinicalTrials.gov number, NCT03434379.) |


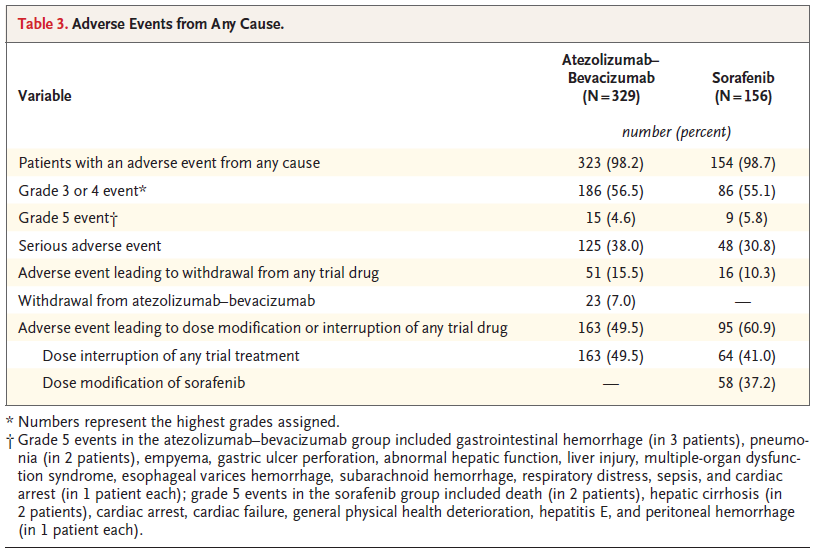

REF. UpToDate 2020.09.08
N Engl J Med. 2020;382(20):1894.
'소화기내과(간) > 간세포암' 카테고리의 다른 글
| TACE, absolute contraindications, relative contraindications (0) | 2020.12.08 |
|---|---|
| Nivolumab plus ipilimumab for advanced hepatocellular cancer previously treated with sorafenib (0) | 2020.09.08 |
| BCLC staging system and therapeutic strategy (0) | 2020.05.09 |
| HCC, Potentially resectable disease & unresectable disease treatment options (0) | 2020.05.08 |
| 2018 간세포암종 진료 가이드라인 [암성 통증의 약물치료] (0) | 2018.09.15 |



