당뇨병에서 권고되는 예방접종. ADA 2023


ADA 2023 추가 내용 3
● 당뇨병 환자에서 인플루엔자 백신 접종 시 live attenuated influenza vaccine은 주의가 필요하며 inactive 또는 recombinant influenza vaccination을 권고한다.
● 두 유형의 폐렴구균백신이 있고 (단백결합백신 = PCV13, PCV15, PCV20과 pneumococcal polysaccharide vaccine=PPSV23) 65세 이상 또는 백신 접종 상태를 모른다면 PCV15 또는 PCV20을 접종하며 만일 PCV15를 접종하였다면 PPSV23를 (1년 이후) 추가로 접종한다. https://blog.naver.com/sjloveu2/222992321615 19-64세이지만 certain underlying risk factors 또는 other medical conditions이 있는 경우도 마찬가지로 적용한다. PPSV23를 먼저 접종하였다면 1년 이후에 PCV15 또는 PCV20를 접종한다. Immunocompromising conditions, cochlear implant, cerebrospinal fluid leak의 경우 PCV15와 PPSV23 사이의 최소 접종 간격은 8주이다.
★★ 현재 우리나라에는 PCV15, PCV20가 있지 않아서 PCV13과 PPSV23 접종 가이드라인에 따릅니다.
- Considerable changes were made in the immunizations subsection to reflect new indications and guidance, particularly for COVID-19 and pneumococcal pneumonia vaccinations, including age-specific recommendations and the bivalent COVID-19 booster.
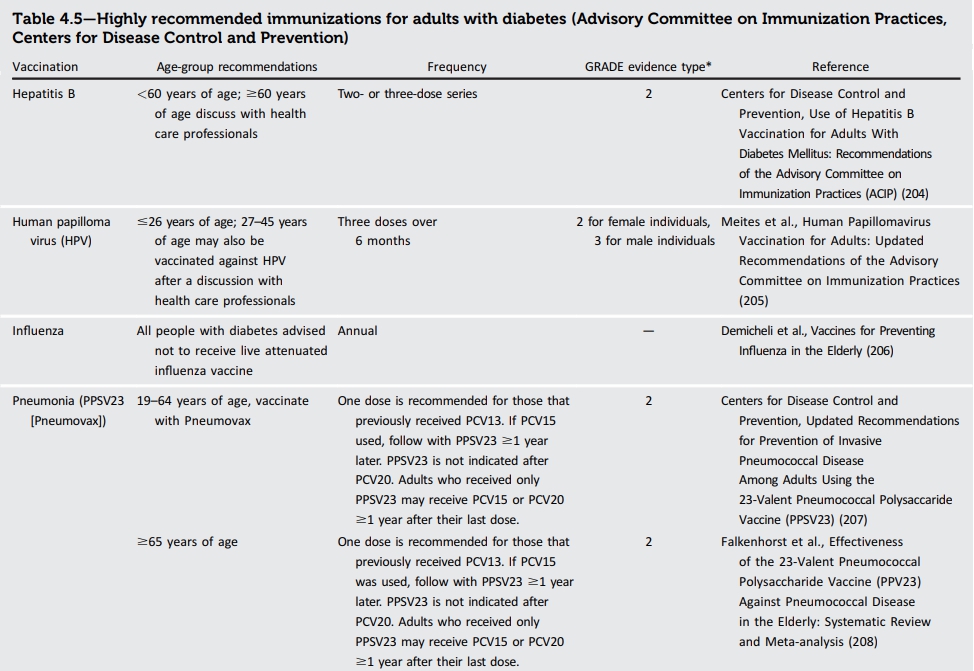

There are two types of vaccines available in the U.S., pneumococcal conjugate vaccines (PCV13, PCV15, and PCV20) and pneumococcal polysaccharide vaccine (PPSV23), with distinct schedules for children and adults. It is recommended that all children receive a four-dose series of PCV13 or PCV15 by 15 months of age. For children with diabetes who have incomplete series by ages 2–5 years, the CDC recommends a catch-up schedule to ensure that these children have four doses. Children with diabetes between 6 and 18 years of age are also advised to receive one dose of PPSV23, preferably after receipt of PCV13. Adults aged ≥65 years whose vaccine status is unknown or who have not received pneumococcal vaccine should receive one dose of PCV15 or PCV20. If PCV15 is used, it should be followed by PPSV23. Adults aged 19–64 years with certain underlying risk factors or other medical conditions whose vaccine status is unknown or who have not received pneumococcal vaccine should receive one dose of PCV15 or PCV20. As for adults aged ≥65 years, if PCV15 is used, it should be followed by PPSV23. The recommended interval between PCV15 and PPSV23 is ≥1 year. If PPSV23 is the only dose received, PCV15 or PCV20 may be given ≥1 year later. For adults with immunocompromising conditions, cochlear implant, or cerebrospinal fluid leak, a minimum interval of 8 weeks can be considered for dosing of PCV15 and PPSV23 when PCV15 has been used. Adults who received PCV13 should follow the previously recommended PPSV23 series. Adults who received only PPSV23 may receive a PCV15 or PCV20 ≥1 year after their last dose.
https://blog.naver.com/sjloveu2/222992321615
[PCV15, PCV20] 성인 폐렴구균백신에 대한 새로운 권고, 2022
US ACIP Adult Immunization Schedule, 2022는 CDC 웹사이트와 Annals of Internal Medicin...
blog.naver.com